Proton therapy
From Wikipedia, the free encyclopedia
Proton therapy is a type of particle therapy which uses a beam of protons to irradiate diseased tissue, most often in the treatment of cancer. The chief advantage of proton therapy is the ability to more precisely localize the radiation dosage when compared with other types of external beam radiotherapy. The development of proton therapy began in the 1950s at accelerator laboratories, and in the last 20 years has expanded to hospital based facilities built specifically to perform this type of treatment.
Contents |
[edit] Description
Proton therapy is a type of external beam radiotherapy using ionizing radiation. During treatment, a particle accelerator is used to target the tumor with a beam of protons.[2][3] These charged particles damage the DNA of cells, ultimately causing their death or interfering with their ability to reproduce. Cancerous cells, because of their high rate of division and their reduced ability to repair damaged DNA, are particularly vulnerable to attack on their DNA.
Due to their relatively large mass, protons have little lateral side scatter in the tissue; the beam does not broaden much, stays focused on the tumor shape and delivers small dose side-effects to surrounding tissue. All protons of a given energy have a certain range; very few protons penetrate beyond that distance.[4] Furthermore, the dose delivered to tissue is maximum just over the last few millimeters of the particle’s range; this maximum is called the Bragg peak.[5]
To treat tumors at greater depths, the proton accelerator must produce a beam with higher energy, typically given in eV or electron volts. Tumors closer to the surface of the body are treated using protons with lower energy. The accelerators used for proton therapy typically produce protons with energies in the range of 70 to 250 MeV (Mega electron Volts: million electron Volts). By adjusting the energy of the protons during application of treatment, the cell damage due to the proton beam is maximized within the tumor itself. Tissues closer to the surface of the body than the tumor receive reduced radiation, and therefore reduced damage. Tissues deeper within the body receive very few protons so that the dosage becomes immeasurably small.[4]
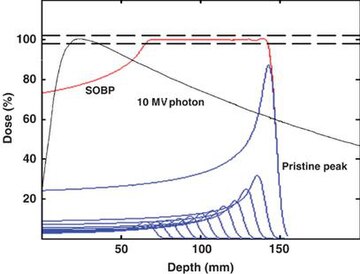
In most treatments, protons of different energies with Bragg peaks at different depths are applied to treat the entire tumor. These Bragg peaks are shown as blue lines in the figure to the left. The total radiation dosage of the protons is called the Spread-Out Bragg Peak (SOBP), shown as a red line in figure to the left. It is important to understand that, while tissues behind or deeper than the tumor receive no radiation from proton therapy, the tissue in front of or shallower than the tumor receive radiation dosage based on the SOBP.
[edit] History
The first suggestion that energetic protons could be an effective treatment method was made by Robert R. Wilson[6] in a paper published in 1946 while he was involved in the design of the Harvard Cyclotron Laboratory (HCL).[7] The first treatments were performed at particle accelerators built for physics research, notably Berkeley Radiation Laboratory in 1954 and at Uppsala in Sweden in 1957. In 1961, a collaboration began between HCL and the Massachusetts General Hospital (MGH) to pursue proton therapy. Over the next 41 years, this program refined and expanded these techniques while treating 9,116 patients[8] before the Cyclotron was shut down in 2002. The first proton therapy center in Western Europe has been in operation at the Paul Scherrer Institute (PSI) in Villigen, Switzerland, since 1984.[8]
Following this pioneering work, the Loma Linda University Medical Center (LLUMC) in Loma Linda, California was built in 1990.[8] Later, The Northeast Proton Therapy Center at Massachusetts General Hospital was brought online, and the HCL treatment program was transferred to it during 2001 and 2002.
[edit] Side effects and risks
Proton therapy is a type of external beam radiotherapy, and shares risks and side effects of other forms of radiation therapy. Proton therapy has been in use for over 40 years, and is a mature treatment technology. However, as with all medical knowledge, understanding of the interaction of radiation (Proton, X-ray, etc.) with tumor and normal tissue is still imperfect.[9][10]
[edit] Application
The types of treatments for which protons are used can be separated into two broad categories.
The first are those for disease sites that favor the delivery of higher doses of radiation, i.e. dose escalation. In some instances dose escalation has been shown to achieve a higher probability of "cure" (i.e. local control) than conventional radiotherapy.[11] These include (but are not limited to) uveal melanoma (ocular tumors), skull base and paraspinal tumors (chondrosarcoma and chordoma), and unresectable sarcomas. In all these cases proton therapy achieves significant improvements in the probability of local control over conventional radiotherapy.[12][13][14]
The second broad class are those treatments where the increased precision of proton therapy is used to reduce unwanted side effects, by limiting the dose to normal tissue. In these cases the tumor dose is the same as that used in conventional therapy, and thus there is no expectation of an increased probability of curing the disease. Instead, the emphasis is on the reduction of the integral dose to normal tissue, and thus a reduction of unwanted effects.[11] Two prominent examples are pediatric neoplasms (such as medulloblastoma) and prostate cancer. In the case of pediatric treatments there is convincing clinical data showing the advantage of sparing developing organs by using protons, and the resulting reduction of long term damage to the surviving child.[15][16]
In the case of prostate cancer the issue is not so clear. Some published studies found a reduction in long term rectal and genitio-urinary damage when treating with proton rather than photons (X-ray) therapy. Others showed the difference is small, and limited to cases where the prostate is particularly close to certain anatomical structures.[17][18] The relatively small improvement found may be the result of inconsistent patient set-up and internal organ movement during treatment, which offsets most of the advantage due to increased precision.[18] [19] [19][20] One source suggests that dose errors around 20% can result from motion errors of just 2.5 mm,[21] and another that prostate motion is between 5–10 mm.[22]
However, the number of cases of prostate cancer diagnosed each year far exceeds those of the other diseases referred to above, and this has led some, but not all, facilities to devote a majority of their treatments slots to prostate treatments. For example two hospital facilities devote roughly 65%[23] and 50%[24] of their proton treatment capacity to prostate cancer, while a third devotes only 7.1%[25]
Current overall world wide numbers are hard to compile, but one example in the literature shows that in 2003 roughly 26% of proton therapy treatments world wide were for prostate cancer.[26]
Proton therapy for ocular (eye) tumors is a special case since this treatment requires only a comparably low energy (about 70 MeV). Owing to this low energy requirement, some particle therapy centers only treat ocular tumors.[8]
[edit] Comparison with other treatment options
The issue of when, whether, and how best to apply this technology is controversial.[27][28][29] As of 2009 it is not yet known whether proton therapy yields better clinical outcomes than other types of radiation therapy for people with many common cancers.[30][31][32][32] Proton therapy is far more expensive than conventional therapy.[28][33] It requires a large capital investment (roughly $100M to $150M[29]) for 2009 technology.[27]
Preliminary results from a three-year 2009 study, including high dose treatments, show very few side effects.[34]
[edit] X-ray radiotherapy

The figure at the top of the page shows how beams of x-rays or beams of protons of different energies penetrate human tissue. A tumor with a sizable thickness is covered by the spread out Bragg peak (SOBP) shown as the red lined distribution in the figure. The SOBP is an overlap of several pristine Bragg peaks (blue lines) at staggered depths.
X-ray therapy may be described as having more "skin sparing potential" than proton therapy: x-ray radiation at the skin and at very small depths is lower than for proton therapy. One study estimates that passively scattered proton fields have a slightly higher entrance dose at the skin (~75%) compared to therapeutic megavoltage photon beams (~60%).[1] X-ray radiation dose falls off gradually, while tissues deeper in the body than the tumor receive essentially no radiation during proton therapy. Thus, x-ray therapy causes less damage to the skin and surface tissues, and proton therapy causes less damage to tissues beyond the target.[3]
[edit] Surgery
The decision to use surgery or proton therapy (or in fact any radiation therapy) is based on the tumor type, stage, and location. In some instances surgery is superior (e.g. cutaneous melanoma), in some instances radiation is superior (e.g. skull base chondrosarcoma), and in some instances they are comparable (e.g. prostate cancer). In some instances, they are used together (e.g. rectal cancer or early stage breast cancer). The benefit of external beam proton radiation lies in the dosimetric difference from external beam x-ray radiation and brachytherapy in cases, where the use of radiation therapy is already indicated, rather than as a direct competition with surgery.[11]
[edit] Treatment centers
At the end of 2008, there were a total of 26 proton therapy centers in Canada, China, England, France, Germany, Italy, Japan, Korea, Russia, South Africa, Sweden, Switzerland, and USA; and over 60000 patients had been treated.[35] One hindrance to universal use of the proton in cancer treatment is the size and cost of the cyclotron or synchrotron equipment necessary. Several industrial teams are working on development of comparatively small cyclotron or synchrotron systems to deliver the proton therapy to patients.[36]
[edit] See also
[edit] References
| Wikimedia Commons has media related to: Proton therapy |
- ^ a b "Proton beam therapy" W P Levin, H Kooy, J S Loeffler and T F DeLaney British Journal of Cancer (2005) 93, 849–854 [1]
- ^ O. Jakel: State of the art in hadron therapy. AIP Conference Proceedings, vol. 958, no.1, 2007, pp. 70-77
- ^ a b "Zap! You're not dead." Economist, 8 September 2007. 384 (8545):13-14
- ^ a b Metz, James (2006-07-31). "Differences Between Protons and X-rays". The Abramson Cancer Center of the University of Pennsylvania. http://www.oncolink.org/treatment/article.cfm?c=9&s=70&id=210. Retrieved 2008-02-04. "the beam then stops, resulting in virtually no radiation to the tissue beyond the target- or no "exit dose""
- ^ Camphausen KA, Lawrence RC. "Principles of Radiation Therapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
- ^ "Radiological Use of Fast Protons", R. R. Wilson, Radiology, 47:487-491 (1946)
- ^ Richard Wilson, "A Brief History of the Harvard University Cyclotrons", Harvard University Press, 2004, pp 9
- ^ a b c d "PTCOG: Particle Therapy Co-Operative Group". Ptcog.web.psi.ch. http://ptcog.web.psi.ch/. Retrieved 2009-09-03.
- ^ Joel E. Tepper, MD, and A. William Blackstock, MD "Randomized Trials and Technology Assessment" Annals of Internal Medicine 151(8) 2009
- ^ Federal Agency for Healthcare Research and Quality "Technical Brief: Particle Beam Radiation Therapies for Cancer" 2009
- ^ a b c R. P. Levy et al,The current status and future directions of heavy charged particle therapy in medicine, AIP Journal, March 2009
- ^ E. B. Hug et al: Proton radiation therapy for chordomas and chondrosarcomas of the skull base, J. Neurosurgery 91, 432-439 (1999)
- ^ E. Gragoudas et al, Evidence-based estimates of outcomes in patients treated for intraocular melenoma", Arch. Ophthalmol.120, 1665-1671 (2002)
- ^ J. E. Munzenrider, N. J. Liebsch, Proton radiotherapy for tumors of the skull base, Strahnlenther. Onkol. 175, 57-63 (1999)
- ^ W. H. St. Clair et al, Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma, Int. J. Radiat. Oncol. Biol. Phys.58, 727-734 (2004)
- ^ D.g. Kirsch and N. J. Tarbell, Conformal radiaiton therapy for chilfhood CNS tumors, The Oncologist 9(4), 442-450 (2004)
- ^ J. D. Slater et al, Proton therapy for prostate cancer; the initial Loma Linda University experience, Int. J. Radiat. Oncol. Biol. Phys 59, 348-352 (2004)
- ^ a b A. L. Zietman et al, Comparisons of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a radomized controlled trial, J. A. M. A. 294(10, 1233-1239 (2005)
- ^ a b R. deCrevoisier et al, "Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy," Int. J. Radiat. Oncol. Biol. Phys. 62(4) 965-973 (2005)
- ^ Lambert et al. "Intrafractional motion during proton beam scanning" Phys. Med. Biol. 50 4853-4862 2005
- ^ Citation Needed - I think it is Philips and Pedroni PMB 1991
- ^ Thomas E. Byrne, "A Review of Prostate Motion with Considerations for the Treatment of Prostate Cancer", Medical Dosimerty 30(3) 2005 pp. 155-161
- ^ Dyk, Jacob, Van (1999). The modern technology of radiation oncology: A Compendium for Medical Physicists and Radiation Oncologists. p. 826: Medical Physics Publishing Corporation. p. 1072. ISBN 0-944838-38-3, 9780944838389. http://books.google.com/books?id=U5JrAAAAMAAJ&. "Proton Patient Summary - Inception Through December 1998...Prostate...2591 64.3%"
- ^ "The Promise of Proton-Beam Therapy". U.S. News and World Report. 2008-04-16. http://health.usnews.com/articles/health/cancer/2008/04/16/the-promise-of-proton-beam-therapy.html?PageNr=1. Retrieved 2008-02-20.
- ^ "Francis H. Burr Proton Therapy Center". http://ptcog.web.psi.ch/PTCOG47/presentations/4_Meeting_Thursday/TDelaneyupdate.pdf.
- ^ J. Sisterson, Ion beam therapy in 2004, Nuclear Instruments and Methods in Physics Research B 241 713-716 (2005)
- ^ a b "EDITORIAL: Randomized Trials and Technology Assessment". Annals of Internal Medicine 151 (8): 583–584. 20 October 2009. http://www.annals.org/cgi/content/short/151/8/583.
- ^ a b BOULTON, GUY (March 30, 2008). "High cost; of high tech; Outlay vs. benefit of expensive medical devices questioned". Milwaukee Journal Sentinel. http://www.highbeam.com/doc/1P2-15594684.html. Retrieved 2009-09-03. ""Despite that controversy, roughly a dozen proton therapy centers have been proposed throughout the country, including northern Illinois""
- ^ a b "The $150 Million Zapper:Does every cancer patient really need proton-beam therapy?". Forbes Magazine. March 16, 2009. http://www.forbes.com/forbes/2009/0316/062_150mil_zapper.html. Retrieved 2009-09-03.
- ^ "Systematic Review: Charged-Particle Radiation Therapy for Cancer". Annals of Internal Medicine 151 (8): 556–565. 20 October 2009. http://www.annals.org/cgi/content/short/151/8/556.
- ^ "Particle Beam Radiation Therapies for Cancer: Policymaker Summary Guide". U.S. Department of Health and Human Services. September 14, 2009. http://effectivehealthcare.ahrq.gov/healthInfo.cfm?infotype=sg&ProcessID=58&DocID=177. Retrieved 2009-10-09.
- ^ a b "Particle Beam Radiation Therapies for Cancer Final Research Review". September 14, 2009. http://effectivehealthcare.ahrq.gov/healthInfo.cfm?infotype=rr&ProcessID=58&DocID=174.
- ^ Feldstein, Dan (Oct 23, 2005). "M.D. Anderson private venture raises questions / Proton-therapy benefits at center won't merit costs of care, some say". Houston Chronicle. http://www.chron.com/CDA/archives/archive.mpl?id=2005_3914112. Retrieved 2009-10-01. ""M.D. Anderson officials estimate that when patients on all types of insurance and payment plans are mixed together, proton delivery will cost an average of $37,000 per patient for prostate treatment, compared with $29,000 for IMRT and $21,000 for standard radiation. The amount excludes doctor fees, which will be roughly the same for each. ""
- ^ Cox, Jeremy (2009-11-23). "UF Proton Therapy Institute study shows positive outcomes". Jacksonville.com. http://jacksonville.com/business/2009-11-23/story/uf_proton_therapy_institute_study_shows_positive_outcomes. Retrieved 2009-12-22.
- ^ "Particle therapy facilities in operation". Particle Therapy Co-Operative Group. http://ptcog.web.psi.ch/ptcentres.html. Retrieved 2008-04-27.
- ^ J.N.A. Matthews: "Accelerators shrink to meet growing demand for proton therapy", Physics Today, März 2009, p. 22
[edit] Further reading
- Greco C, Wolden S. Current status of radiotherapy with proton and light ion beams. Cancer. 2007 Apr 1;109(7):1227-38 PMID 17326046
- "Use of Protons for Radiotherapy", A.M. Koehler, Proc. of the Symposium on Pion and Proton Radiotherapy, Nat. Accelerator Lab., (1971)
- "Protons in Radiation Therapy: comparative Dose Distributions for Protons, Photons and Electrons, A.M. Koehler, W.M. Preston, Radiology, 104(1):191-195 (1972)
- "Bragg Peak Proton Radiosurgery for Arteriovenous Malformation of the Brain" R.N. Kjelberg, presented at First Int. Seminar on the Use of Proton Beams in Radiation Therapy, Moskow (1977)
- "Fractionated Proton Radiation Therapy of Cranial and Intracrainial Tumors" Austin-Seymor, M.J. Munzenrider, et al. Am.J.of Clinical Oncology 13(4):327-330 (1990)
- "Proton Radiotherapy", Hartford, Zietman, et al. in Radiotheraputic Management of Carcinoma of the Prostate, A. D'Amico and G.E. Hanks. London,UK, Arnold Publishers: 61-72 (1999)

