| Acute myeloid leukemia | |
|---|---|
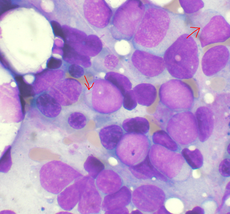
Bone marrow aspirate showing acute myeloid leukemia. Arrows indicate Auer rods.
|
|
| Classification and external resources | |
| Specialty | Hematology and oncology |
| ICD-10 | C92.0 |
| ICD-9 | 205.0 |
| ICD-O: | M9861/3 |
| OMIM | 602439 |
| DiseasesDB | 203 |
| MedlinePlus | 000542 |
| eMedicine | med/34 |
| NCI | Acute myeloid leukemia |
| Patient UK | Acute myeloid leukemia |
| MeSH | D015470 |
Acute myeloid leukemia (AML), also known as acute myelogenous leukemia or acute nonlymphocytic leukemia (ANLL), is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal white blood cells that accumulate in the bone marrow and interfere with the production of normal blood cells. AML is the most common acute leukemia affecting adults, and its incidence increases with age. Although AML is a relatively rare disease, accounting for approximately 1.2% of cancer deaths in the United States,[1] its incidence is expected to increase as the population ages.
The symptoms of AML are caused by replacement of normal bone marrow with leukemic cells, which causes a drop in red blood cells, platelets, and normal white blood cells. These symptoms include fatigue, shortness of breath, easy bruising and bleeding, and increased risk of infection. Several risk factors and chromosomal abnormalities have been identified, but the specific cause is not clear. As an acute leukemia, AML progresses rapidly and is typically fatal within weeks or months if left untreated.
AML has several subtypes; treatment and prognosis varies among subtypes. Five-year survival varies from 15–70%, and relapse rate varies from 33–78%, depending on subtype. AML is treated initially with chemotherapy aimed at inducing a remission; patients may go on to receive additional chemotherapy or a hematopoietic stem cell transplant. Recent research into the genetics of AML has resulted in the availability of tests that can predict which drug or drugs may work best for a particular patient, as well as how long that patient is likely to survive.
Signs and symptomsEdit
Most signs and symptoms of AML are caused by the replacement of normal blood cells with leukemic cells. A lack of normal white blood cell production makes the patient susceptible to infections; while the leukemic cells themselves are derived from white blood cell precursors, they have no infection-fighting capacity.[2] A drop in red blood cell count (anemia) can cause fatigue, paleness, and shortness of breath. A lack of platelets can lead to easy bruising or bleeding with minor trauma.
The early signs of AML are often vague and nonspecific, and may be similar to those of influenza or other common illnesses. Some generalized symptoms include fever, fatigue, weight loss or loss of appetite, shortness of breath, anemia, easy bruising or bleeding, petechiae (flat, pin-head sized spots under the skin caused by bleeding), bone and joint pain, and persistent or frequent infections.[2]
Enlargement of the spleen may occur in AML, but it is typically mild and asymptomatic. Lymph node swelling is rare in AML, in contrast to acute lymphoblastic leukemia. The skin is involved about 10% of the time in the form of leukemia cutis. Rarely, Sweet's syndrome, a paraneoplastic inflammation of the skin, can occur with AML.[2]
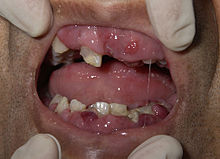
Some patients with AML may experience swelling of the gums because of infiltration of leukemic cells into the gum tissue. Rarely, the first sign of leukemia may be the development of a solid leukemic mass or tumor outside of the bone marrow, called a chloroma. Occasionally, a person may show no symptoms, and the leukemia may be discovered incidentally during a routine blood test.[3]
CausesEdit
A number of risk factors for developing AML have been identified, including: other blood disorders, chemical exposures, ionizing radiation, and genetics.
PreleukemiaEdit
"Preleukemic" blood disorders, such as myelodysplastic syndrome or myeloproliferative disease, can evolve into AML; the exact risk depends on the type of MDS/MPS.[4]
Chemical exposureEdit
Exposure to anticancer chemotherapy, in particular alkylating agents, can increase the risk of subsequently developing AML. The risk is highest about three to five years after chemotherapy.[5] Other chemotherapy agents, specifically epipodophyllotoxins and anthracyclines, have also been associated with treatment-related leukemia. These treatment-related leukemias are often associated with specific chromosomal abnormalities in the leukemic cells.[6]
Occupational chemical exposure to benzene and other aromatic organic solvents is controversial as a cause of AML. Benzene and many of its derivatives are known to be carcinogenic in vitro. While some studies have suggested a link between occupational exposure to benzene and increased risk of AML,[7] others have suggested the attributable risk, if any, is slight.[8]
RadiationEdit
High amounts of ionizing radiation exposure can increase the risk of AML. Survivors of the atomic bombings of Hiroshima and Nagasaki had an increased rate of AML,[9] as did radiologists exposed to high levels of X-rays prior to the adoption of modern radiation safety practices.[10]
GeneticsEdit
A hereditary risk for AML appears to exist. Multiple cases of AML developing in a family at a rate higher than predicted by chance alone have been reported.[11][12][13][14] Several congenital conditions may increase the risk of leukemia; the most common is probably Down syndrome, which is associated with a 10- to 18-fold increase in the risk of AML.[15]
DiagnosisEdit
The first clue to a diagnosis of AML is typically an abnormal result on a complete blood count. While an excess of abnormal white blood cells (leukocytosis) is a common finding, and leukemic blasts are sometimes seen, AML can also present with isolated decreases in platelets, red blood cells, or even with a low white blood cell count (leukopenia).[16] While a presumptive diagnosis of AML can be made via examination of the peripheral blood smear when there are circulating leukemic blasts, a definitive diagnosis usually requires an adequate bone marrow aspiration and biopsy.
Marrow or blood is examined via light microscopy, as well as flow cytometry, to diagnose the presence of leukemia, to differentiate AML from other types of leukemia (e.g. acute lymphoblastic leukemia - ALL), and to classify the subtype of disease (see below). A sample of marrow or blood is typically also tested for chromosomal abnormalities by routine cytogenetics or fluorescent in situ hybridization. Genetic studies may also be performed to look for specific mutations in genes such as FLT3, nucleophosmin, and KIT, which may influence the outcome of the disease.[17]
Cytochemical stains on blood and bone marrow smears are helpful in the distinction of AML from ALL, and in subclassification of AML. The combination of a myeloperoxidase or Sudan black stain and a nonspecific esterase stain will provide the desired information in most cases. The myeloperoxidase or Sudan black reactions are most useful in establishing the identity of AML and distinguishing it from ALL. The nonspecific esterase stain is used to identify a monocytic component in AMLs and to distinguish a poorly differentiated monoblastic leukemia from ALL.[18]
The diagnosis and classification of AML can be challenging, and should be performed by a qualified hematopathologist or hematologist. In straightforward cases, the presence of certain morphologic features (such as Auer rods) or specific flow cytometry results can distinguish AML from other leukemias; however, in the absence of such features, diagnosis may be more difficult.[19]
According to the widely used WHO criteria, the diagnosis of AML is established by demonstrating involvement of more than 20% of the blood and/or bone marrow by leukemic myeloblasts.[20] The French–American–British (FAB) classification is a bit more stringent, requiring a blast percentage of at least 30% in bone marrow (BM) or peripheral blood (PB) for the diagnosis of AML.[21] AML must be carefully differentiated from "preleukemic" conditions such as myelodysplastic or myeloproliferative syndromes, which are treated differently.
Because acute promyelocytic leukemia (APL) has the highest curability and requires a unique form of treatment, it is important to quickly establish or exclude the diagnosis of this subtype of leukemia. Fluorescent in situ hybridization performed on blood or bone marrow is often used for this purpose, as it readily identifies the chromosomal translocation [t(15;17)(q22;q12);] that characterizes APL. There is also a need to molecularly detect the presence of PML/RARA fusion protein, which is an oncogenic product of that translocation. [22]
The two most commonly used classification schemata for AML are the older French-American-British (FAB) system and the newer World Health Organization (WHO) system.
World Health OrganizationEdit
The World Health Organization (WHO) classification of acute myeloid leukemia attempts to be more clinically useful and to produce more meaningful prognostic information than the FAB criteria. Each of the WHO categories contains numerous descriptive subcategories of interest to the hematopathologist and oncologist; however, most of the clinically significant information in the WHO schema is communicated via categorization into one of the subtypes listed below.
The WHO subtypes of AML are:[23]
| Name | Description | ICD-O |
|---|---|---|
| Acute myeloid leukemia with recurrent genetic abnormalities | Includes:
|
Multiple |
| AML with myelodysplasia-related changes | This category includes patients who have had a prior documented myelodysplastic syndrome (MDS) or myeloproliferative disease (MPD) that then has transformed into AML, or who have cytogenetic abnormalities characteristic for this type of AML (with previous history of MDS or MPD that has gone unnoticed in the past, but the cytogenetics is still suggestive of MDS/MPD history). This category of AML occurs most often in elderly patients and often has a worse prognosis. Includes:
|
M9895/3 |
| Therapy-related myeloid neoplasms | This category includes patients who have had prior chemotherapy and/or radiation and subsequently develop AML or MDS. These leukemias may be characterized by specific chromosomal abnormalities, and often carry a worse prognosis. | M9920/3 |
| Myeloid sarcoma | This category includes myeloid sarcoma. | |
| Myeloid proliferations related to Down syndrome | This category includes so-called "transient abnormal myelopoiesis" and "Myeloid leukemia associated with Down syndrome" | |
| Blastic plasmacytoid dendritic cell neoplasm | This category includes so-called "blastic plasmacytoid dendritic cell neoplasm" | |
| AML not otherwise categorized | Includes subtypes of AML that do not fall into the above categories | M9861/3 |
Acute leukemias of ambiguous lineage (also known as mixed phenotype or biphenotypic acute leukemia) occur when the leukemic cells can not be classified as either myeloid or lymphoid cells, or where both types of cells are present.
French-American-BritishEdit
The French-American-British (FAB) classification system divides AML into eight subtypes, M0 through to M7, based on the type of cell from which the leukemia developed and its degree of maturity. This is done by examining the appearance of the malignant cells with light microscopy and/or by using cytogenetics to characterize any underlying chromosomal abnormalities. The subtypes have varying prognoses and responses to therapy. Although the WHO classification (see above) may be more useful, the FAB system is still widely used.
Eight FAB subtypes were proposed in 1976.[24]
| Type | Name | Cytogenetics | Percentage of adult AML patients |
|---|---|---|---|
| M0 | acute myeloblastic leukemia, minimally differentiated | 5%[25] | |
| M1 | acute myeloblastic leukemia, without maturation | 15%[25] | |
| M2 | acute myeloblastic leukemia, with granulocytic maturation | t(8;21)(q22;q22), t(6;9) | 25%[25] |
| M3 | promyelocytic, or acute promyelocytic leukemia (APL) | t(15;17) | 10%[25] |
| M4 | acute myelomonocytic leukemia | inv(16)(p13q22), del(16q) | 20%[25] |
| M4eo | myelomonocytic together with bone marrow eosinophilia | inv(16), t(16;16) | 5%[25] |
| M5 | acute monoblastic leukemia (M5a) or acute monocytic leukemia (M5b) | del (11q), t(9;11), t(11;19) | 10%[25] |
| M6 | acute erythroid leukemias, including erythroleukemia (M6a) and very rare pure erythroid leukemia (M6b) | 5%[25] | |
| M7 | acute megakaryoblastic leukemia | t(1;22) | 5%[25] |
The morphologic subtypes of AML also include rare types not included in the FAB system, such as acute basophilic leukemia, which was proposed as a ninth subtype, M8, in 1999.[26]
PathophysiologyEdit
The malignant cell in AML is the myeloblast. In normal hematopoiesis, the myeloblast is an immature precursor of myeloid white blood cells; a normal myeloblast will gradually mature into a mature white blood cell. In AML, though, a single myeloblast accumulates genetic changes which "freeze" the cell in its immature state and prevent differentiation.[27] Such a mutation alone does not cause leukemia; however, when such a "differentiation arrest" is combined with other mutations which disrupt genes controlling proliferation, the result is the uncontrolled growth of an immature clone of cells, leading to the clinical entity of AML.[28]
Much of the diversity and heterogeneity of AML stems is because leukemic transformation can occur at a number of different steps along the differentiation pathway.[29] Modern classification schemes for AML recognize the characteristics and behavior of the leukemic cell (and the leukemia) may depend on the stage at which differentiation was halted.
Specific cytogenetic abnormalities can be found in many patients with AML; the types of chromosomal abnormalities often have prognostic significance.[30] The chromosomal translocations encode abnormal fusion proteins, usually transcription factors whose altered properties may cause the "differentiation arrest".[31] For example, in acute promyelocytic leukemia, the t(15;17) translocation produces a PML-RARα fusion protein which binds to the retinoic acid receptor element in the promoters of several myeloid-specific genes and inhibits myeloid differentiation.[32]
The clinical signs and symptoms of AML result from the growth of leukemic clone cells, which tends to displace or interfere with the development of normal blood cells in the bone marrow.[33] This leads to neutropenia, anemia, and thrombocytopenia. The symptoms of AML are, in turn, often due to the low numbers of these normal blood elements. In rare cases, patients can develop a chloroma, or solid tumor of leukemic cells outside the bone marrow, which can cause various symptoms depending on its location.[2]
TreatmentEdit
First-line treatment of AML consists primarily of chemotherapy, and is divided into two phases: induction and postremission (or consolidation) therapy. The goal of induction therapy is to achieve a complete remission by reducing the number of leukemic cells to an undetectable level; the goal of consolidation therapy is to eliminate any residual undetectable disease and achieve a cure.[34] Hematopoietic stem cell transplantation is usually considered if induction chemotherapy fails or after a patient relapses, although transplantation is also sometimes used as front-line therapy for patients with high-risk disease.
InductionEdit
All FAB subtypes except M3 are usually given induction chemotherapy with cytarabine (ara-C) and an anthracycline (most often daunorubicin).[35] This induction chemotherapy regimen is known as "7+3" (or "3+7"), because the cytarabine is given as a continuous IV infusion for seven consecutive days while the anthracycline is given for three consecutive days as an IV push. Up to 70% of patients will achieve a remission with this protocol.[36] Other alternative induction regimens, including high-dose cytarabine alone, FLAG-like regimens or investigational agents, may also be used.[37][38] Because of the toxic effects of therapy, including myelosuppression and an increased risk of infection, induction chemotherapy may not be offered to the very elderly, and the options may include less intense chemotherapy or palliative care.
The M3 subtype of AML, also known as acute promyelocytic leukemia (APL), is almost universally treated with the drug all-trans-retinoic acid (ATRA) in addition to induction chemotherapy, usually an anthracycline.[39][40][41] Care must be taken to prevent disseminated intravascular coagulation (DIC), complicating the treatment of APL when the promyelocytes release the contents of their granules into the peripheral circulation. APL is eminently curable, with well-documented treatment protocols.
The goal of the induction phase is to reach a complete remission. Complete remission does not mean the disease has been cured; rather, it signifies no disease can be detected with available diagnostic methods.[35] Complete remission is obtained in about 50%–75% of newly diagnosed adults, although this may vary based on the prognostic factors described above.[42] The length of remission depends on the prognostic features of the original leukemia. In general, all remissions will fail without additional consolidation therapy.[43]
ConsolidationEdit
Even after complete remission is achieved, leukemic cells likely remain in numbers too small to be detected with current diagnostic techniques. If no further postremission or consolidation therapy is given, almost all patients will eventually relapse.[44] Therefore, more therapy is necessary to eliminate nondetectable disease and prevent relapse — that is, to achieve a cure.
The specific type of postremission therapy is individualized based on a patient's prognostic factors (see above) and general health. For good-prognosis leukemias (i.e. inv(16), t(8;21), and t(15;17)), patients will typically undergo an additional three to five courses of intensive chemotherapy, known as consolidation chemotherapy.[45][46] For patients at high risk of relapse (e.g. those with high-risk cytogenetics, underlying MDS, or therapy-related AML), allogeneic stem cell transplantation is usually recommended if the patient is able to tolerate a transplant and has a suitable donor. The best postremission therapy for intermediate-risk AML (normal cytogenetics or cytogenetic changes not falling into good-risk or high-risk groups) is less clear and depends on the specific situation, including the age and overall health of the patient, the patient's personal values, and whether a suitable stem cell donor is available.[46]
For patients who are not eligible for a stem cell transplant, immunotherapy with a combination of histamine dihydrochloride (Ceplene) and interleukin 2 (Proleukin) after the completion of consolidation has been shown to reduce the absolute relapse risk by 14%, translating to a 50% increase in the likelihood of maintained remission.[47]
Relapsed AMLEdit
For patients with relapsed AML, the only proven potentially curative therapy is a hematopoietic stem cell transplant, if one has not already been performed.[48][49][50] In 2000, the monoclonal antibody-linked cytotoxic agent gemtuzumab ozogamicin (Mylotarg) was approved in the United States for patients aged more than 60 years with relapsed AML who are not candidates for high-dose chemotherapy.[51] This drug was voluntarily withdrawn from the market by its manufacturer, Pfizer in 2010.[52]
Since treatment options for relapsed AML are so limited, palliative care may be offered.
Clinical trialsEdit
Patients with relapsed AML who are not candidates for stem cell transplantion, or who have relapsed after a stem cell transplant, may be offered treatment in a clinical trial, as conventional treatment options are limited. Agents under investigation include cytotoxic drugs such as clofarabine, as well as targeted therapies, such as farnesyl transferase inhibitors, decitabine, and inhibitors of MDR1 (multidrug-resistance protein).
Relapsed APLEdit
For relapsed acute promyelocytic leukemia (APL), arsenic trioxide has been tested in trials and approved by the US FDA. Like ATRA, arsenic trioxide does not work with other subtypes of AML.[53]
PrognosisEdit
Acute myeloid leukemia is a curable disease; the chance of cure for a specific patient depends on a number of prognostic factors.[54]
CytogeneticsEdit
The single most important prognostic factor in AML is cytogenetics, or the chromosomal structure of the leukemic cell. Certain cytogenetic abnormalities are associated with very good outcomes (for example, the (15;17) translocation in acute promyelocytic leukemia). About half of AML patients have "normal" cytogenetics; they fall into an intermediate risk group. A number of other cytogenetic abnormalities are known to associate with a poor prognosis and a high risk of relapse after treatment.[55][56][57]
The first publication to address cytogenetics and prognosis was the MRC trial of 1998:[58]
| Risk Category | Abnormality | 5-year survival | Relapse rate |
|---|---|---|---|
| Good | t(8;21), t(15;17), inv(16) | 70% | 33% |
| Intermediate | Normal, +8, +21, +22, del(7q), del(9q), Abnormal 11q23, all other structural or numerical changes | 48% | 50% |
| Poor | -5, -7, del(5q), Abnormal 3q, Complex cytogenetics | 15% | 78% |
Later, the Southwest Oncology Group and Eastern Cooperative Oncology Group[59] and, later still, Cancer and Leukemia Group B published other, mostly overlapping lists of cytogenetics prognostication in leukemia.[60]
Myelodysplastic syndromeEdit
AML which arises from a pre-existing myelodysplastic syndrome (MDS) or myeloproliferative disease (so-called secondary AML) has a worse prognosis, as does treatment-related AML arising after chemotherapy for another previous malignancy. Both of these entities are associated with a high rate of unfavorable cytogenetic abnormalities.[61][62][63]
Other prognostic markersEdit
In some studies, age >60 years and elevated lactate dehydrogenase level were also associated with poorer outcomes.[64] As with most forms of cancer, performance status (i.e. the general physical condition and activity level of the patient) plays a major role in prognosis as well.
GenotypeEdit
FLT3 internal tandem duplications (ITDs) have been shown to confer a poorer prognosis in AML.[65] Treating these patients with more aggressive therapy, such as stem-cell transplantation in first remission, has not been shown to enhance long-term survival. ITDs of FLT3 may be associated with leukostasis.[66] In 2012 FLT3 inhibitor quizartinib had good phase II trial results in AML patients with FLT3-ITD mutations.
Researchers are investigating the clinical significance of c-KIT mutations in AML.[67] These are prevalent, and clinically relevant because of the availability of tyrosine kinase inhibitors, such as imatinib and sunitinib that can block the activity of c-KIT pharmacologically.
Other genes being investigated as prognostic factors or therapeutic targets include CEBPA, BAALC, ERG, and NPM1.
Expectation of cureEdit
Cure rates in clinical trials have ranged from 20–45%;[68][69] although clinical trials often include only younger patients and those able to tolerate aggressive therapies. The overall cure rate for all patients with AML (including the elderly and those unable to tolerate aggressive therapy) is likely lower. Cure rates for promyelocytic leukemia can be as high as 98%.[70]
EpidemiologyEdit
Acute myeloid leukemia is a relatively rare cancer. There are approximately 10,500 new cases each year in the United States, and the incidence rate has remained stable from 1995 through 2005. AML accounts for 1.2% of all cancer deaths in the United States.[1]
The incidence of AML increases with age; the median age at diagnosis is 63 years. AML accounts for about 90% of all acute leukemias in adults, but is rare in children.[1] The rate of therapy-related AML (that is, AML caused by previous chemotherapy) is rising; therapy-related disease currently accounts for about 10–20% of all cases of AML.[71] AML is slightly more common in men, with a male-to-female ratio of 1.3:1.[72]
There is some geographic variation in the incidence of AML. In adults, the highest rates are seen in North America, Europe, and Oceania, while adult AML is rarer in Asia and Latin America.[73][74] In contrast, childhood AML is less common in North America and India than in other parts of Asia.[75] These differences may be due to population genetics, environmental factors, or a combination of the two.
UKEdit
AML accounts for 34% of all leukaemia cases in the UK, and around 2,900 people were diagnosed with the disease in 2011.[76]
HistoryEdit
The first published description of a case of leukemia in medical literature dates to 1827, when French physician Alfred-Armand-Louis-Marie Velpeau described a 63-year-old florist who developed an illness characterized by fever, weakness, urinary stones, and substantial enlargement of the liver and spleen. Velpeau noted the blood of this patient had a consistency "like gruel", and speculated the appearance of the blood was due to white corpuscles.[77] In 1845, a series of patients who died with enlarged spleens and changes in the "colors and consistencies of their blood" was reported by the Edinburgh-based pathologist J.H. Bennett; he used the term "leucocythemia" to describe this pathological condition.[78]
The term "leukemia" was coined by Rudolf Virchow, the renowned German pathologist, in 1856. As a pioneer in the use of the light microscope in pathology, Virchow was the first to describe the abnormal excess of white blood cells in patients with the clinical syndrome described by Velpeau and Bennett. As Virchow was uncertain of the etiology of the white blood cell excess, he used the purely descriptive term "leukemia" (Greek: "white blood") to refer to the condition.[79]
Further advances in the understanding of acute myeloid leukemia occurred rapidly with the development of new technology. In 1877, Paul Ehrlich developed a technique of staining blood films which allowed him to describe in detail normal and abnormal white blood cells. Wilhelm Ebstein introduced the term "acute leukemia" in 1889 to differentiate rapidly progressive and fatal leukemias from the more indolent chronic leukemias.[80] The term "myeloid" was coined by Franz Ernst Christian Neumann in 1869, as he was the first to recognize white blood cells were made in the bone marrow (Greek: µυєλός, myelos = (bone) marrow) as opposed to the spleen. The technique of bone marrow examination to diagnose leukemia was first described in 1879 by Mosler.[81] Finally, in 1900, the myeloblast, which is the malignant cell in AML, was characterized by Otto Naegeli, who divided the leukemias into myeloid and lymphocytic.[82][83]
In 2008, AML became the first cancer genome to be fully sequenced. DNA extracted from leukemic cells were compared to unaffected skin.[84] The leukemic cells contained acquired mutations in several genes that had not previously been associated with the disease.
PregnancyEdit
Leukemia is rarely associated with pregnancy, affecting only about 1 in 10,000 pregnant women.[85] How it is handled depends primarily on the type of leukemia. Acute leukemias normally require prompt, aggressive treatment, despite significant risks of pregnancy loss and birth defects, especially if chemotherapy is given during the developmentally sensitive first trimester.[85]
ReferencesEdit
- ^ a b c Jemal A, Thomas A, Murray T, Thun M (2002). "Cancer statistics, 2002". CA Cancer J Clin 52 (1): 23–47. doi:10.3322/canjclin.52.1.23. PMID 11814064.
- ^ a b c d Hoffman, Ronald (2005). Hematology: Basic Principles and Practice (4th. ed.). St. Louis, Mo.: Elsevier Churchill Livingstone. pp. 1074–75. ISBN 0-443-06629-9.
- ^ Abeloff, Martin (2004). Clinical Oncology (3rd. ed.). St. Louis, Mo.: Elsevier Churchill Livingstone. p. 2834. ISBN 0-443-06629-9.
- ^ Sanz GF, Sanz MA, Vallespí T, Cañizo MC, Torrabadella M, García S, Irriguible D, San Miguel JF (1989). "Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: a multivariate analysis of prognostic factors in 370 patients". Blood 74 (1): 395–408. PMID 2752119.
- ^ Le Beau MM, Albain KS, Larson RA, Vardiman JW, Davis EM, Blough RR, Golomb HM, Rowley JD (1986). "Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7". J Clin Oncol 4 (3): 325–45. PMID 3950675.
- ^ Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, McCabe NR, Kobayashi H, Ziemin-van der Poel S, Kaneko Y, Morgan R, Sandberg AA (1993). "Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations". N Engl J Med 329 (13): 909–14. doi:10.1056/NEJM199309233291302. PMID 8361504.
- ^ Austin H, Delzell E, Cole P (1988). "Benzene and leukemia. A review of the literature and a risk assessment". Am J Epidemiol 127 (3): 419–39. PMID 3277397.
- ^ Linet, MS. The Leukemias: Epidemiologic Aspects. Oxford University Press, New York 1985.
- ^ Bizzozero OJ, Johnson KG, Ciocco A (1966). "Radiation-related leukemia in Hiroshima and Nagasaki, 1946–1964. I. Distribution, incidence and appearance time". N Engl J Med 274 (20): 1095–101. doi:10.1056/NEJM196605192742001. PMID 5932020.
- ^ Yoshinaga S, Mabuchi K, Sigurdson AJ, Doody MM, Ron E (2004). "Cancer risks among radiologists and radiologic technologists: review of epidemiologic studies". Radiology 233 (2): 313–21. doi:10.1148/radiol.2332031119. PMID 15375227.
- ^ Taylor GM, Birch JM (1996). "The hereditary basis of human leukemia". In Henderson ES, Lister TA, Greaves MF. Leukemia (6th ed.). Philadelphia: WB Saunders. p. 210. ISBN 0-7216-5381-2.
- ^ Horwitz M, Goode EL, Jarvik GP (1996). "Anticipation in familial leukemia". Am. J. Hum. Genet. 59 (5): 990–8. PMC 1914843. PMID 8900225.
- ^ Crittenden LB (1961). "An interpretation of familial aggregation based on multiple genetic and environmental factors". Ann. N. Y. Acad. Sci. 91 (3): 769–80. Bibcode:1961NYASA..91..769C. doi:10.1111/j.1749-6632.1961.tb31106.x. PMID 13696504.
- ^ Horwitz M (1997). "The genetics of familial leukemia". Leukemia 11 (8): 1347–59. doi:10.1038/sj.leu.2400707. PMID 9264391.
- ^ Evans DI, Steward JK (1972). "Down's syndrome and leukaemia". Lancet 2 (7790): 1322. doi:10.1016/S0140-6736(72)92704-3. PMID 4117858.
- ^ Abeloff, Martin et al. (2004), p. 2834.
- ^ Baldus CD, Mrózek K, Marcucci G, Bloomfield CD (June 2007). "Clinical outcome of de novo acute myeloid leukaemia patients with normal cytogenetics is affected by molecular genetic alterations: a concise review". Br. J. Haematol. 137 (5): 387–400. doi:10.1111/j.1365-2141.2007.06566.x. PMID 17488484.
- ^ Vardiman JW, Harris NL, Brunning RD (2002). "The World Health Organization (WHO) classification of the myeloid neoplasms". Blood 100 (7): 2292–302. doi:10.1182/blood-2002-04-1199. PMID 12239137.
- ^ Abeloff, Martin et al. (2004), p. 2835.
- ^ Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD (1999). "The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997". Ann Oncol 10 (12): 1419–32. doi:10.1023/A:1008375931236. PMID 10643532.
- ^ Amin HM, Yang Y, Shen Y, Estey EH, Giles FJ, Pierce SA, Kantarjian HM, O'Brien SM, Jilani I, Albitar M (2005). "Having a higher blast percentage in circulation than bone marrow: Clinical implications in myelodysplastic syndrome and acute lymphoid and myeloid leukemias". Leukemia 19 (9): 1567–1572. doi:10.1038/sj.leu.2403876. PMID 16049515.
- ^ Grimwade D, Howe K, Langabeer S, Davies L, Oliver F, Walker H, Swirsky D, Wheatley K, Goldstone A, Burnett A, Solomon E (1996). "Establishing the presence of the t(15;17) in suspected acute promyelocytic leukaemia: cytogenetic, molecular and PML immunofluorescence assessment of patients entered into the M.R.C. ATRA trial. M.R.C. Adult Leukaemia Working Party". Br J Haematol 94 (3): 557–73. doi:10.1046/j.1365-2141.1996.d01-1004.x. PMID 8790159.
- ^ Falini B, Tiacci E, Martelli MP, Ascani S, Pileri SA (October 2010). "New classification of acute myeloid leukemia and precursor-related neoplasms: changes and unsolved issues". Discov Med 10 (53): 281–92. PMID 21034669.
- ^ Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (1976). "Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group". Br J Haematol 33 (4): 451–8. doi:10.1111/j.1365-2141.1976.tb03563.x. PMID 188440.
- ^ a b c d e f g h i Seiter, Karen; Jules, E Harris (20 May 2011). "Acute Myeloid Leukemia Staging". Retrieved 26 August 2011.
- ^ Duchayne E, Demur C, Rubie H, Robert A, Dastugue N (1999). "Diagnosis of acute basophilic leukemia". Leuk Lymphoma 32 (3–4): 269–78. doi:10.3109/10428199909167387. PMID 10037024.
- ^ Fialkow PJ (1976). "Clonal origin of human tumors". Biochim. Biophys. Acta 458 (3): 283–321. doi:10.1016/0304-419X(76)90003-2. PMID 1067873.
- ^ Fialkow PJ, Janssen JW, Bartram CR (1 April 1991). "Clonal remissions in acute nonlymphocytic leukemia: evidence for a multistep pathogenesis of the malignancy" (PDF). Blood 77 (7): 1415–7. PMID 2009365.
- ^ Bonnet D, Dick JE (1997). "Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell". Nat. Med. 3 (7): 730–7. doi:10.1038/nm0797-730. PMID 9212098.
- ^ Abeloff, Martin et al. (2004), pp. 2831–32.
- ^ Greer JP et al., ed. (2004). Wintrobe's Clinical Hematology (11th ed.). Philadelphia: Lippincott, Williams, and Wilkins. pp. 2045–2062. ISBN 0-7817-3650-1.
- ^ Melnick A, Licht JD (15 May 1999). "Deconstructing a disease: RARα, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia". Blood 93 (10): 3167–215. PMID 10233871.
- ^ Abeloff, Martin et al. (2004), p. 2828.
- ^ Acute myeloid leukemia at Mount Sinai Hospital
- ^ a b Abeloff, Martin et al. (2004), pp. 2835–39.
- ^ Bishop JF (1997). "The treatment of adult acute myeloid leukemia". Semin Oncol 24 (1): 57–69. PMID 9045305.
- ^ Weick JK, Kopecky KJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP, Bickers JN, Hynes HE, Welborn JL, Simon SR, Grever M (15 October 1996). "A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study". Blood 88 (8): 2841–51. PMID 8874180.
- ^ Bishop JF, Matthews JP, Young GA, Szer J, Gillett A, Joshua D, Bradstock K, Enno A, Wolf MM, Fox R, Cobcroft R, Herrmann R, Van Der Weyden M, Lowenthal RM, Page F, Garson OM, Juneja S (1 March 1996). "A randomized study of high-dose cytarabine in induction in acute myeloid leukemia". Blood 87 (5): 1710–7. PMID 8634416.
- ^ Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wang ZY (1 August 1988). "Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia". Blood 72 (2): 567–72. PMID 3165295.
- ^ Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, Wiernik PH (1997). "All-trans-retinoic acid in acute promyelocytic leukemia". N. Engl. J. Med. 337 (15): 1021–8. doi:10.1056/NEJM199710093371501. PMID 9321529.
- ^ Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, Gardin C, Makhoul PC, Travade P, Solary E, Fegueux N, Bordessoule D, Miguel JS, Link H, Desablens B, Stamatoullas A, Deconinck E, Maloisel F, Castaigne S, Preudhomme C, Degos L (15 August 1999). "A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group". Blood 94 (4): 1192–200. PMID 10438706.
- ^ Estey EH (2002). "Treatment of acute myelogenous leukemia". Oncology (Williston Park) 16 (3): 343–52, 355–6; discussion 357, 362, 365–6. PMID 15046392.
- ^ Cassileth PA, Harrington DP, Hines JD, Oken MM, Mazza JJ, McGlave P, Bennett JM, O'Connell MJ (1988). "Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia". J Clin Oncol 6 (4): 583–7. PMID 3282032.
- ^ Cassileth PA, Harrington DP, Hines JD, Oken MM, Mazza JJ, McGlave P, Bennett JM, O'Connell MJ (1988). "Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia". J. Clin. Oncol. 6 (4): 583–7. PMID 3282032.
- ^ Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei E (1994). "Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B". N. Engl. J. Med. 331 (14): 896–903. doi:10.1056/NEJM199410063311402. PMID 8078551.
- ^ a b Appelbaum FR, Baer MR, Carabasi MH, Coutre SE, Erba HP, Estey E, Glenn MJ, Kraut EH, Maslak P, Millenson M, Miller CB, Saba HI, Stone R, Tallman MS (2000). "NCCN Practice Guidelines for Acute Myelogenous Leukemia". Oncology (Williston Park, N.Y.) 14 (11A): 53–61. PMID 11195419.
- ^ Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann WK, Hogge DE, Nilsson B, Or R, Romero AI, Rowe JM, Simonsson B, Spearing R, Stadtmauer EA, Szer J, Wallhult E, Hellstrand K (July 2006). "Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial". Blood 108 (1): 88–96. doi:10.1182/blood-2005-10-4073. PMID 16556892.
- ^ Abeloff, Martin et al. (2004), pp. 2840–41.
- ^ Appelbaum FR (2001). "Editorial: Who should be transplanted for AML?". Leukemia 15 (4): 680–2. doi:10.1038/sj/leu/2402074. PMID 11368380.
- ^ Appelbaum FR (2002). "Keynote address: hematopoietic cell transplantation beyond first remission". Leukemia 16 (2): 157–9. doi:10.1038/sj.leu.2402345. PMID 11840278.
- ^ Sievers EL, Larson RA, Stadtmauer EA, Estey E, Löwenberg B, Dombret H, Karanes C, Theobald M, Bennett JM, Sherman ML, Berger MS, Eten CB, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR (1 July 2001). "Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse". J. Clin. Oncol. 19 (13): 3244–54. PMID 11432892.
- ^ http://www.pfizer.com/files/products/mylotarg_hcp_letter.pdf
- ^ Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutré S, Dahlberg S, Ellison R, Warrell RP (15 September 2001). "United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia". J. Clin. Oncol. 19 (18): 3852–60. PMID 11559723.
- ^ Estey EH (2001). "Prognostic factors in acute myelogenous leukemia". Leukemia 15 (4): 670–2. doi:10.1038/sj/leu/2402057. PMID 11368376.
- ^ Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, Rees JK, Stevens RF, Walker H (1999). "A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties". Br J Haematol 107 (1): 69–79. doi:10.1046/j.1365-2141.1999.01684.x. PMID 10520026.
- ^ Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR (15 December 2000). "Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study". Blood 96 (13): 4075–83. PMID 11110676.
- ^ Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PR, Moore JO, Stone RM, Mayer RJ, Feldman EJ, Davey FR, Schiffer CA, Larson RA, Bloomfield CD (2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood 100 (13): 4325–36. doi:10.1182/blood-2002-03-0772. PMID 12393746.
- ^ Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A (1 October 1998). "The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties". Blood 92 (7): 2322–33. PMID 9746770.
- ^ Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR (2000). "Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study". Blood 96 (13): 4075–83. PMID 11110676.
- ^ Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PR, Moore JO, Stone RM, Mayer RJ, Feldman EJ, Davey FR, Schiffer CA, Larson RA, Bloomfield CD (2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood 100 (13): 4325–36. doi:10.1182/blood-2002-03-0772. PMID 12393746.
- ^ Thirman MJ, Larson RA (1996). "Therapy-related myeloid leukemia". Hematol Oncol Clin North Am 10 (2): 293–320. doi:10.1016/S0889-8588(05)70340-3. PMID 8707757.
- ^ Rowley JD, Golomb HM, Vardiman JW (1 October 1981). "Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease". Blood 58 (4): 759–67. PMID 7272506.
- ^ Lee JT, Lee JD, Choe KO, Yang WI (2002). "Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia". Blood 99 (6): 1909–12. doi:10.1182/blood.V99.6.1909. PMID 1877259.
- ^ Haferlach T, Schoch C, Löffler H, Gassmann W, Kern W, Schnittger S, Fonatsch C, Ludwig WD, Wuchter C, Schlegelberger B, Staib P, Reichle A, Kubica U, Eimermacher H, Balleisen L, Grüneisen A, Haase D, Aul C, Karow J, Lengfelder E, Wörmann B, Heinecke A, Sauerland MC, Büchner T, Hiddemann W (2003). "Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies". J. Clin. Oncol. 21 (2): 256–65. doi:10.1200/JCO.2003.08.005. PMID 12525517.
- ^ Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T, Haferlach T, Hiddemann W (2002). "Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease". Blood 100 (1): 59–66. doi:10.1182/blood.V100.1.59. PMID 12070009.
- ^ Thornton KA, Levis M (2007). "Images in clinical medicine. FLT3 Mutation and acute myelogenous leukemia with leukostasis". N. Engl. J. Med. 357 (16): 1639. doi:10.1056/NEJMicm064764. PMID 17942876.
- ^ Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, Vukosavljevic T, Perrotti D, Vardiman JW, Carroll AJ, Kolitz JE, Larson RA, Bloomfield CD (2006). "Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study". J. Clin. Oncol. 24 (24): 3904–11. doi:10.1200/JCO.2006.06.9500. PMID 16921041.
- ^ Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, Willman C, Hurd DD, Bennett JM, Blume KG, Head DR, Wiernik PH (1998). "Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission". N. Engl. J. Med. 339 (23): 1649–56. doi:10.1056/NEJM199812033392301. PMID 9834301.
- ^ Matthews JP, Bishop JF, Young GA, Juneja SK, Lowenthal RM, Garson OM, Cobcroft RG, Dodds AJ, Enno A, Gillett EA, Hermann RP, Joshua DE, Ma DD, Szer J, Taylor KM, Wolf M, Bradstock KF (2001). "Patterns of failure with increasing intensification of induction chemotherapy for acute myeloid leukaemia". Br. J. Haematol. 113 (3): 727–36. doi:10.1046/j.1365-2141.2001.02756.x. PMID 11380464.
- ^ Sanz MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, Díaz-Mediavilla J, Fioritoni G, González JD, Liso V, Esteve J, Ferrara F, Bolufer P, Bernasconi C, Gonzalez M, Rodeghiero F, Colomer D, Petti MC, Ribera JM, Mandelli F (15 August 2000). "Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups". Blood 96 (4): 1247–53. PMID 10942364.
- ^ Leone G, Mele L, Pulsoni A, Equitani F, Pagano L (1 October 1999). "The incidence of secondary leukemias". Haematologica 84 (10): 937–45. PMID 10509043.
- ^ Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001). "Cancer statistics, 2001". CA Cancer J Clin 51 (1): 15–36. doi:10.3322/canjclin.51.1.15. PMID 11577478.
- ^ Linet MS (1985). "The leukemias: Epidemiologic aspects.". In Lilienfeld AM. Monographs in Epidemiology and Biostatistics. New York: Oxford University Press. p. I. ISBN 0-19-503448-1.
- ^ Aoki K, Kurihars M, Hayakawa N (1992). Death Rates for Malignant Neoplasms for Selected Sites by Sex and Five-Year Age Group in 33 Countries 1953–57 to 1983–87. Nagoya, Japan: University of Nagoya Press, International Union Against Cancer.
- ^ Bhatia S, Neglia JP (1995). "Epidemiology of childhood acute myelogenous leukemia". J. Pediatr. Hematol. Oncol. 17 (2): 94–100. doi:10.1097/00043426-199505000-00002. PMID 7749772.
- ^ "Acute myeloid leukaemia (AML) statistics". Cancer Research UK. Retrieved 27 October 2014.
- ^ Hoffman et al. 2005, pg 1071
- ^ Bennett JH (1845). "Two cases of hypertrophy of the spleen and liver, in which death took place from suppuration of blood". Edinburgh Med Surg J 64: 413.
- ^ Virchow, R (1856). "Die Leukämie". In Virchow R. Gesammelte Abhandlungen zur Wissenschaftlichen Medizin (in German). Frankfurt: Meidinger. p. 190.
- ^ Ebstein W (1889). "Über die acute Leukämie und Pseudoleukämie". Deutsch Arch Klin Med 44: 343.
- ^ Mosler F (1876). "Klinische Symptome und Therapie der medullären Leukämie". Berl Klin Wochenschr 13: 702.
- ^ Naegeli O (1900). "Über rothes Knochenmark und Myeloblasten". Deutsch Med Wochenschr 26 (18): 287. doi:10.1055/s-0029-1203820.
- ^ Wang ZY (2003). "Ham-Wasserman Lecture: Treatment of Acute Leukemia by Inducing Differentiation and Apoptosis". Hematology 2003 (1): 1–13. doi:10.1182/asheducation-2003.1.1. PMID 14633774.
- ^ Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, Dooling D, Dunford-Shore BH, McGrath S, Hickenbotham M, Cook L, Abbott R, Larson DE, Koboldt DC, Pohl C, Smith S, Hawkins A, Abbott S, Locke D, Hillier LW, Miner T, Fulton L, Magrini V, Wylie T, Glasscock J, Conyers J, Sander N, Shi X, Osborne JR, Minx P, Gordon D, Chinwalla A, Zhao Y, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson M, Baty J, Ivanovich J, Heath S, Shannon WD, Nagarajan R, Walter MJ, Link DC, Graubert TA, DiPersio JF, Wilson RK (2008). "DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome". Nature 456 (7218): 66–72. doi:10.1038/nature07485. PMC 2603574. PMID 18987736.
- ^ a b Shapira T, Pereg D, Lishner M (September 2008). "How I treat acute and chronic leukemia in pregnancy". Blood Rev. 22 (5): 247–59. doi:10.1016/j.blre.2008.03.006. PMID 18472198.
External linksEdit
- Acute myeloid leukemia at DMOZ
- GeneReviews/NIH/NCBI/UW entry on Familial Acute Myeloid Leukemia (AML) with Mutated CEBPA
- PDQ statement on AML for health professionals at National Cancer Institute
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||